Chemical equilibrium
Overall concentration changes
Increasing the concentration of the reactants is equivalent to increasing the pressure.
\( K_c \)
Procedure
- When the gas coefficients on both sides of the reaction equation are equal, \( V \) can be canceled, that is one can use \( n \) instead of \(c \).
NCEE

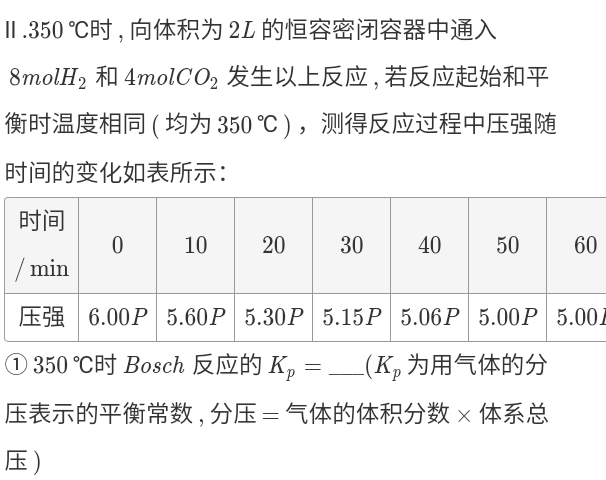
\( K_p \)
Procedure
- Sometimes \( p \) is proportional to \( n \) (\(p V = n R t \))
- \( K_p = \frac{p(C)^c p(D)^d}{p(A)^a p(B)^b} = \frac{(p \cdot x(C))^c (p \cdot x(D))^d}{(p \cdot x(A))^a (p \cdot x(B))^b} = p^{(c + d) - (a + b)} \frac{x(C)^c x(D)^d}{x(A)^a x(B)^b} \) (\(x \) is Mole fraction)
Constant pressure
NCEE
1
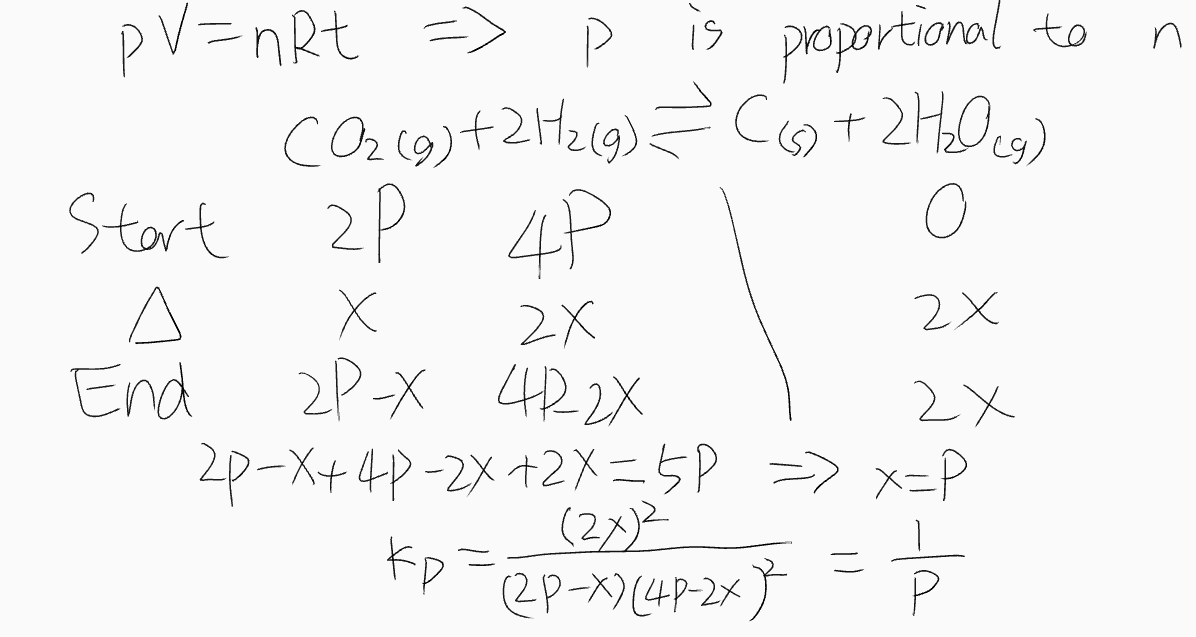
\( N_2 + 3H_2 \rightleftharpoons 2NH_3 \)
\( \frac{3-x}{6-2x} \) = 2