Maximum number of coplanar atoms
The only case where all atoms are not coplanar is saturated carbon atoms. A saturated carbon atom is a carbon atom that has only single bonds between other atoms.
Procedure
- Locate saturated carbon atoms
- Cut out 2 bonds in the principle of maximizing the number of atoms remaining
Exercise
How many maximum number of atoms are coplanar in
Extra: Asymmetric carbon
some 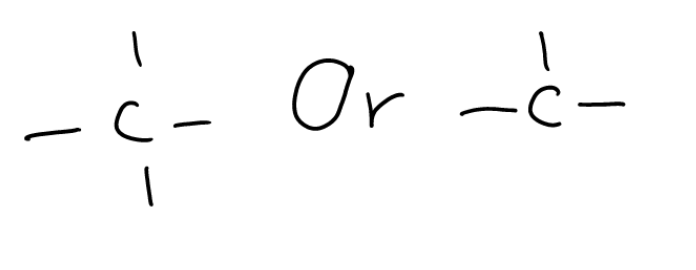 with branch chains
with branch chains

